Trial Pal and PFS are two of the tools developed by Integra IT that have been used in the Covid 19 project being conducted in Brazil. The ease with which they collect and organize information makes them allies in this type of scientific development.
The role of Integra IT in the Covid 19 project
The Integra IT technology is at the service of the Phase 3 study of the Fiocruz SARS-CoV-2 vaccine in Brazil. This project involves the participation of 10,000 volunteers who are monitored for one year after the application of the ChAdOx1 nCoV-19 biological.
The project is being carried out in Brazil, a Latin American nation with 211 million inhabitants. Its geographic extension, as well as its population density, allows research to be conducted and conclusions to be drawn based on the crossing of variables derived from factors such as climate, regional conditions, and common diseases, among others.
The Covid 19 project has participants from five states: Bahia, Sao Paulo, Rio de Janeiro, Rio Grande do Sul, Rio Grande do Norte, all bordering the Atlantic Ocean; each of these territories has one or two sites that are in charge of the clinical research of the participants.
Storing, harnessing, and processing the information that these 10,000 people provide over the course of a year is no easy task; in order to achieve this, technological tools are needed to enable volunteers to provide details, of their symptomatology, for example, in a clear and easy manner, immediately notifying the sites in order to expedite the timely detection of COVID-19 cases.
Likewise, those in charge of receiving the information can organize it so that the sponsor can be notified and make the most of it according to the study objectives.
The Integra IT tools in the Covid 19 project
Integra IT, with its TrialPal and PFS products, has ensured that the process of delivering and processing information is convenient, secure, and optimal for both parties.

Integra IT has a trained team ready to provide timely support for any problem that may occur, whether for operational or technological issues involving TrialPal or PFS.
TrialPal and FPS contributions to the study
Data collection through the tools offered by Integra IT has mainly allowed the optimization of processes and the detection of Covid-19 cases almost in real time. Evitar Tasks that could take up to three days now only require hours, without affecting quality.
Another of the contributions is taking advantage of the information. Although sponsors have access to a wide variety of data, this data is often not used to its full potential as there is no filter to identify and enhance it as another way to contribute to the studies.
In short, the information arrives, but it is not sufficiently broken down. The Integra IT tools not only help in this scenario, but they can also identify errors and the respective solutions to these errors..
A success story in efficiency
The group in charge of the analysis of the Covid 19 project focused on Sao Paulo was made up of a large team that was in charge of attending the entire flow of subjects participating in the study; however, in certain months, the presence of all the collaborators of the site team was not necessary.
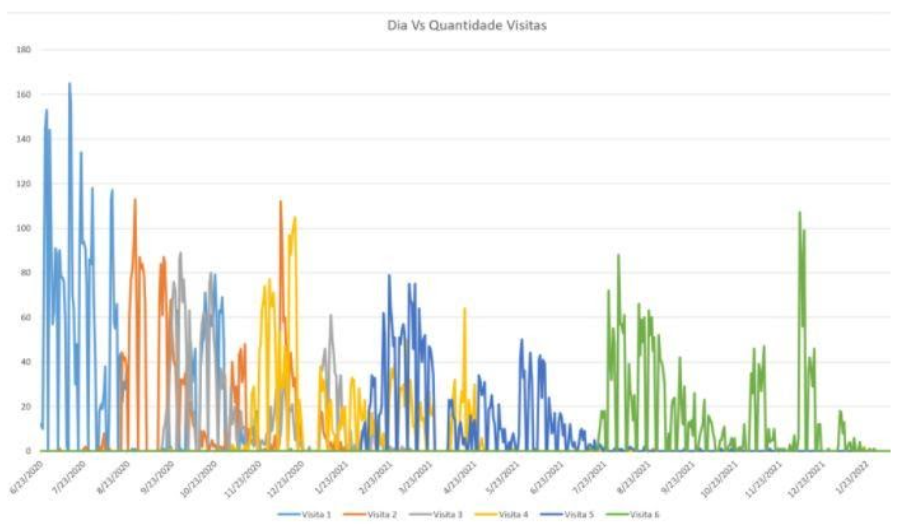
Thanks to the PFS tool, it was possible to detect when more or less people were required, based on the participation flow of the project collaborators, and thus better plan the demand and balance workloads to better serve the participants without any delays, as shown below.
Based on the information collected, we were able to create this graph which shows the number of visits compared to the day and identify the moments when more or less logistical support was needed.
Learnings thanks to the Integra IT tools
- Timely identification of the COVID-19 cases once the participant reported symptoms and immediate action was taken by the sites - in more than 90% of the cases.
- Tasks that take up to three days for a site can be done in hours, with the same quality.
- It contributes to the collaboration and communication between the sites, the CRO and the Sponsor by centralizing the data, and each user has access to real-time information on the reports and symptoms sent by participants.
- Cost reduction and better cost utilization.
- The ability to devise new ways of working to optimize the study.
- Improved processes thanks to technology.
- It guarantees the security of the information.
- It reduces workloads.
- It allows focusing efforts on more important issues.