TrialPal ePRO + PFS (Patient Follow Up System Site CTMS)
This is an intuitive and easy-to-use tool that allows monitoring of study participants and the study in general, combined with an easy to use app created to keep track of your participants.

Integra IT TrialPal Modules
We have a product that contains a differentiating factor that generates value, offering solutions for monitoring and defining processes. It informs you in the form of a dashboard, containing the information collected. Alerts and notifications are generated, and there is a projection that helps decision taking.
11
MILLION REPORTS
48
PARTICIPANTS
12
YEARS EXPERIENCE
15
COUNTRIES
Join our Partnership Program
With an initial study discount!
Join by clicking on the Apply Now button
e-Diary
Diaries are used in clinical trials where investigators want to gather information after each vaccination or medication given. With this module, the information reported by patients is shared in real-time, to enable site staff to know what is happening with each patient
We also improve the quality of information, creating validations inside the App which minimizes typing errors.

Customizable
We can configure any form in order to be aligned with the protocol.
User-friendly
It asks simple questions and sends the required information in less than a minute.
No internet - No Problem
The app saves the report on the device and once it is back online, all the pending data will be sent automatically.
Traceability
All the information from subjects is encrypted and compliant with all the industry guidelines.
Vigilant-e
The Vigilant-e module allows clinical trial participants to report actions with information to the study site. It is ideal for surveillance studies where participants must report their health status periodically for long periods of time.
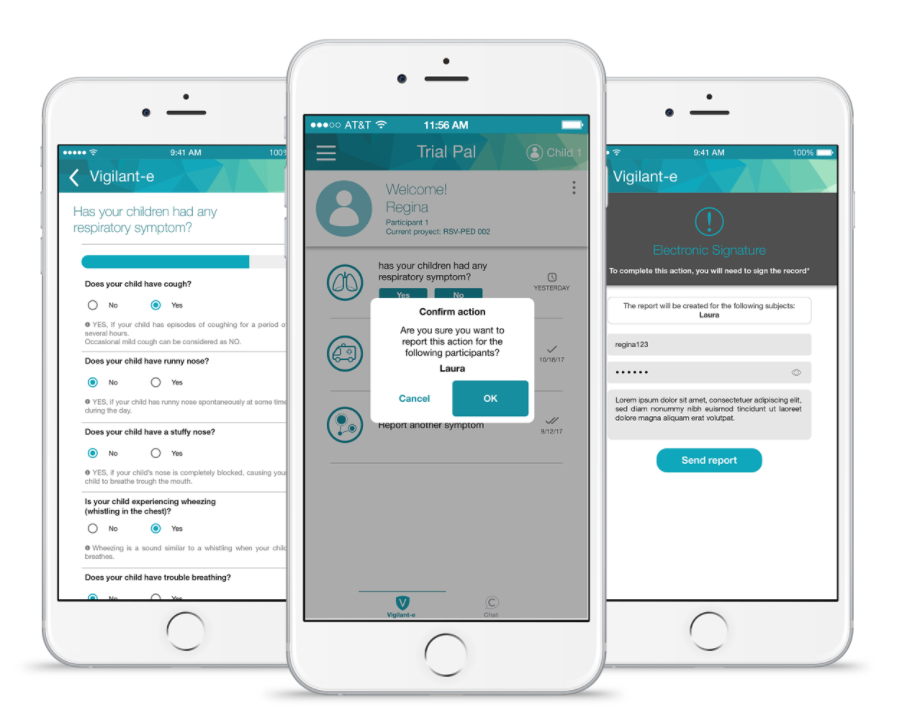
Symptoms
Contact Request
Hospitalizations
Chat
The Chat module is designed to improve communication between the participants and the site. It provides all the tools of traceability and safety ensuring that all conversations related to the study remain registered in our databases.
Site users are able to review the conversations related to study participants or legal representatives. It is the best way to stay in touch with patients.
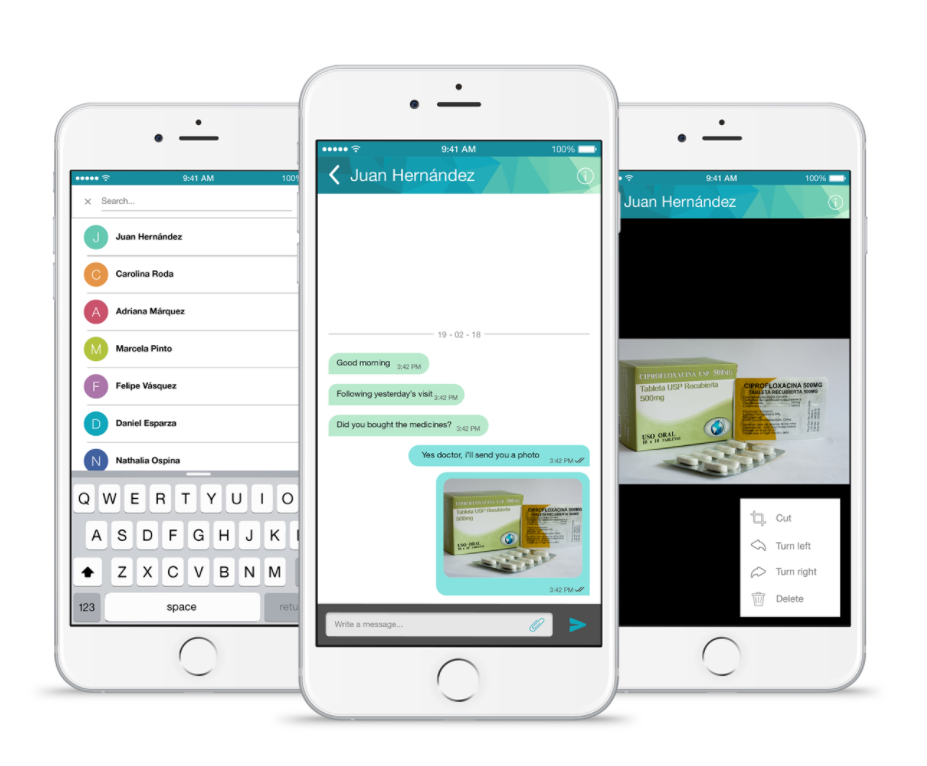
BENEFITS OF THE CHAT MODULE
Benefits of TrialPal
Reliable
Real-time information
Configurable
User-friendly
Off-line functions
Traceability
Mobile App
Requirements
We develop solutions as a service, take a look at how they work.
CONTACT USPFS II
Patient Follow up System
PFS is a web system developed in order to collect information from clinical trials. Therefore it is the application where all the information collected by our Trial Pal is centralized.
PFS Product is a specialized software based on highly reliable and available platforms that guarantees an excellent service performance without interruptions, integrating security standards, and guideliness such as CFR Part 11 from the FDA. This system is designed mainly for the Sites and will allow efficient management of subjects and the trial.

Through PFS, a Site can manage interactively and in real time, all the information of the subjects, including receiving email alerts indicating any important event for the study. Likewise, it is a key aspect for maintaining high adherence of patients during the study since it has detailed tracking information such as: what subjects have not reported, what the last visit made was and by whom, when the next visit, next call is, etc.
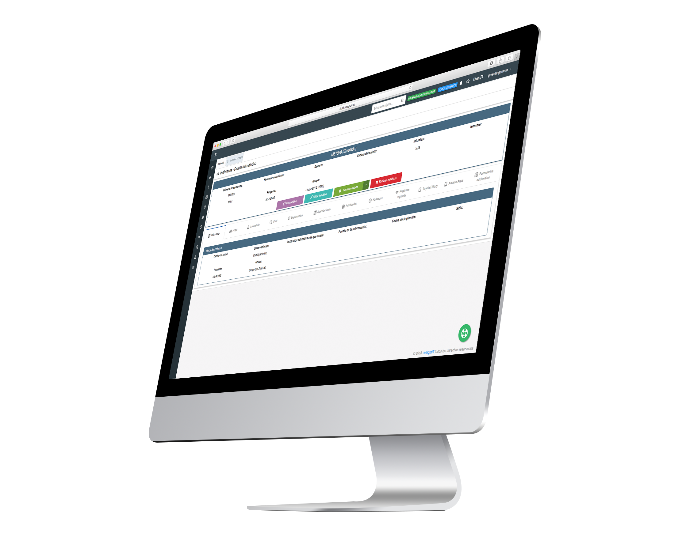
Subject Module
This is where all patient information is stored (contact information, parent information, subject number, etc). All information collected is handled with roles, and profiles that will have access according to their privileges, to see more or less detail of subjects; always guaranteeing the confidentiality and integrity of information.
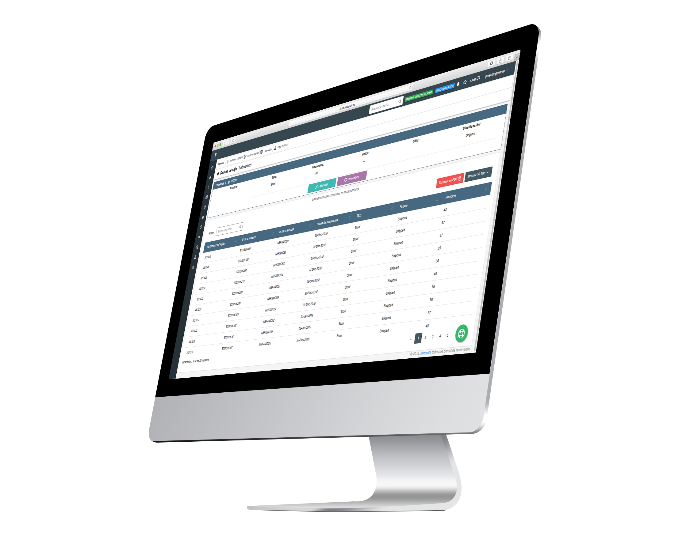
Subject Log Module
This module presents a very simple-to-use functionality that records all the contacts a site has had with the study subjects. Thus, a consolidated tracking log for each subject. Team members know who the last person was to contact the subject and when this happened.
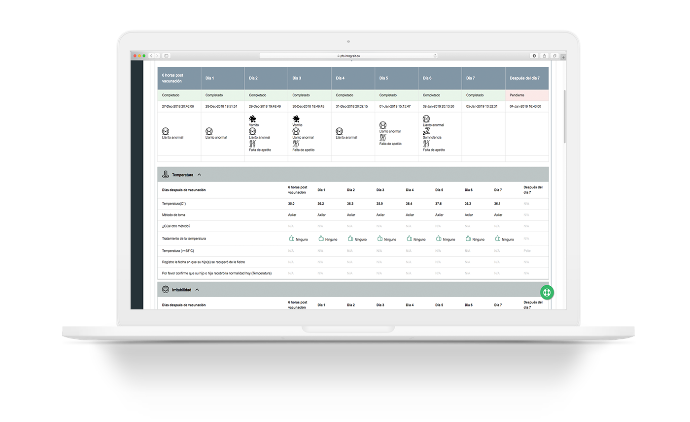
PFS-Lab Sample
This module helps to manage the laboratory samples generated during the study such as stool and blood samples. The Site can configure different states that these samples can be in, and each of them is used to create a form that contains information about the state, including changes. It also helps manage the location where samples are stored.
We have developed an interface with CDC laboratory systems for more efficient communication between study sites and CDC.
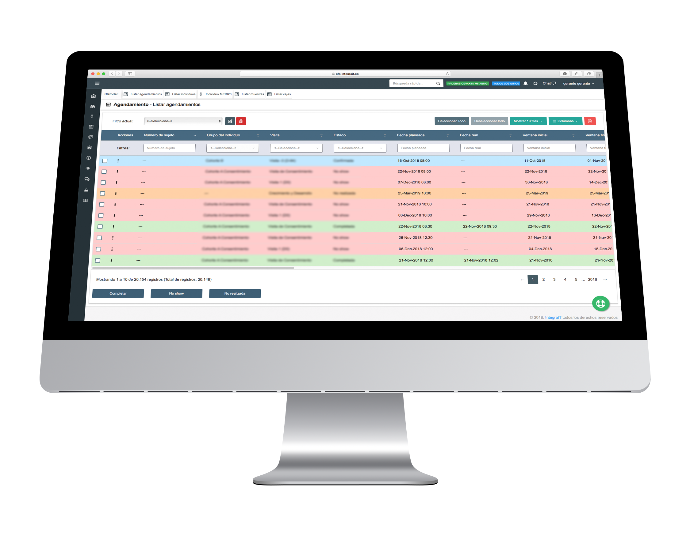
PFS-Schedule
This module allows control of the subject visit agenda. It presents a calendar view to show all study visits. The main feature is that this module helps sites to calculate study visit windows, including holiday management, for accurate and real planning.
PFS-Candidate
This module allows mass uploading of information from different sources of potential subjects for a new study. It also allows the scheduling of initial study visits. Once a candidate has passed all the enrollment process, they can be promoted to subject / patient within the system.
PFS-Activities
The activities module allows the user to complete and/or monitor the progress of the various activities associated with a subject that is participating in a project or clinical trial. Within the module, site staff can perform the following actions: Start Activities; List Activities; Assign Activities; View Statistics; Download Activity Reports; Perform Activities.
If you want to know more about how our systems are validated and comply with 21 CFR Part 11
Success Story
Vax Trials
Vax Trials is a regional leader Clinical Research Organization that provides innovative solutions, managing and monitoring activities of vaccine clinical trials across Latin America.
Our solutions helped Vax Trials to improve on its efficiency and management of data in every one of its clinical studies, helping it to commit less mistakes and gather all the information digitally and securely.