
Multiple Solutions to your CRO
Our solutions range from a modular STS to a CTMS that will allow us to have real time control of the clinics and all the studies.
- TrialPal ePRO + Site CTMS
- STS (Study Tracking System)
- CRF (Case Report Form System & Data Management Services)
- Data Management Services
Want to know how to get our solutions?
Schedule a meeting with us!
Join our Partnership Program
With an initial study discount!
Join by clicking on the Apply Now button
Our Services For CROs
We have created a set of services and solutions to help CROs adapt to a digital world and transform with the accompaniment needed to change and gain insights and control to comply with each clinical trial objective.
We develop solutions as a service, take a look at how they work.
CONTACT US
TrialPal ePRO + PFS (Patient Follow Up System Site CTMS)
TrialPal + PFS II
An intuitive and easy-to-use tool that allows monitoring of study participants and the study in general, combined with an easy to use app created to keep track of your patients.

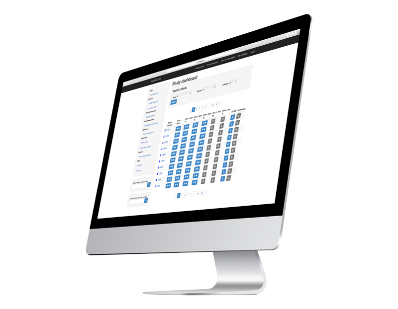
- We have a product that contains a differentiating factor that generates value, offering solutions for monitoring and defining processes. It informs you in the form of a dashboard, containing the information collected. Alerts and notifications are generated, and there is a projection that helps decision taking.
- PFS, allows the collection of all the data necessary for decision making, and the visualization of the traceability in changes, guaranteeing transparency of the information provided.
-
Study Tracking System
STS
This system is designed so that CROs (Contract Research Organizations) can optimize visits to research centers.
Our STS system allows you to keep a record of the visits of the CRAs (Clinical Research Associate) to each of the sites that develop a clinical study.
Within the STS we have electronic signatures so that monitors and Project Managers can certify that all processes are carried out according to best practices.

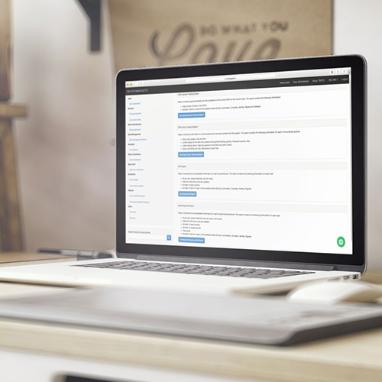
Its main
characteristics are:
-
Creation and approval of reports.
-
PDF downloads
-
Scheduling of monitoring visits
-
Registering and downloading reports
If you want to know more about how our systems are validated and comply with 21 CFR Part 11
Do you want to talk and know more about any solution?
schedule a meetingCase Report
Form System
CRF & Data Management Services
Our WEB Information System (Case Report Form System) is used to enter and monitor the information of a clinical study efficiently, quickly and safely. It can be configured according to study and protocol requirement.
The data we store is protected so that only authorized persons can access it. Additionally, our data is backed up by backup copies that are saved every 6 hours.

When working with our CRF, you will have total confidence in where your data is and who accesses it, since we work with processes with high quality standards and security. We have a team that will monitor your requirements and provide solutions to them.
You will have access to documents such as manuals, configuration of forms and validation of data. You can contact us to show you a DEMO and test the tool.
Study Cases
Grateful customers
As Integra we care about the satisfaction of our clients and the satisfactory performance of each of our solutions, always providing them with the best support and listening to every one of the necesities they may have.
Here are some of our study cases.
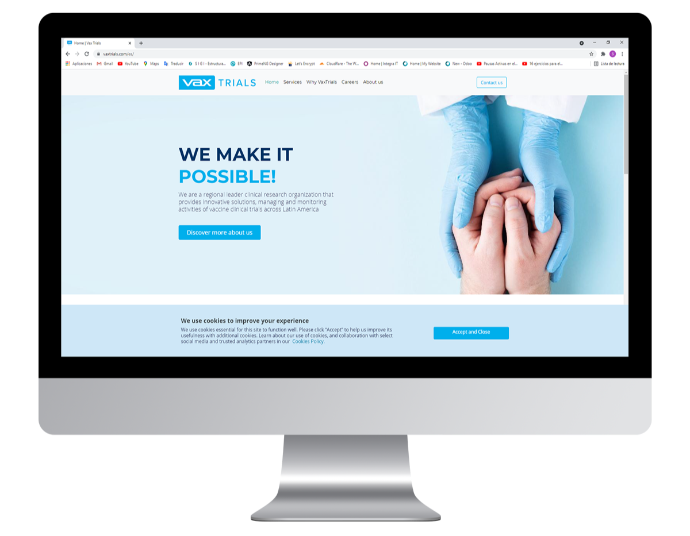
Vax Trials
Our solutions helped Vax Trials to improve on their efficiency and management of data in every one of their clinical studies, helping them to commit less mistakes, and gather all their information digitally and securely.


