Data Management Services
Fast and Accurate Insights

Join our Partnership Program
With an initial study discount!
Join by clicking on the Apply Now button
-
Allow early problem solving.
-
Send notifications and alerts to sites.
-
Ensure Data Quality.
-
Receive better outcomes.
-
Give you speed and accuracy.
-
Ensure better and faster decision making.
-
Include two dictionaries (MeDRA and WHO) that give advice regarding the standard medical and medicines terminology.
Data Management over our CRF takes advantage of automatic checks, and automatic tasks to ensure speed and error free processes.
Our CRF is already integrated and synchronized with our PFS (Patient Follow Up System), used by sites to manage a clinical trial; thereby reducing errors and costs in data transfer or digitalization.
Data management Experience
-
Polio Phase 2 study - Colombia
-
Polio Phase 2 study - Panama, Dominican Republic
-
Real World Evidence (RWE) birth control - Peru
-
Breast Cancer (RWE) - Colombia
-
mCRPC (RWE) - Colombia
-
Norovirus Observational study - Panama
-
Sexual Arousal Disorder Phase 3 - Colombia
-
Real World Evidence (RWE) Asthma - Colombia, Argentina, Persian Gulf, India, Chile, Turkey, Saudi Arabia)
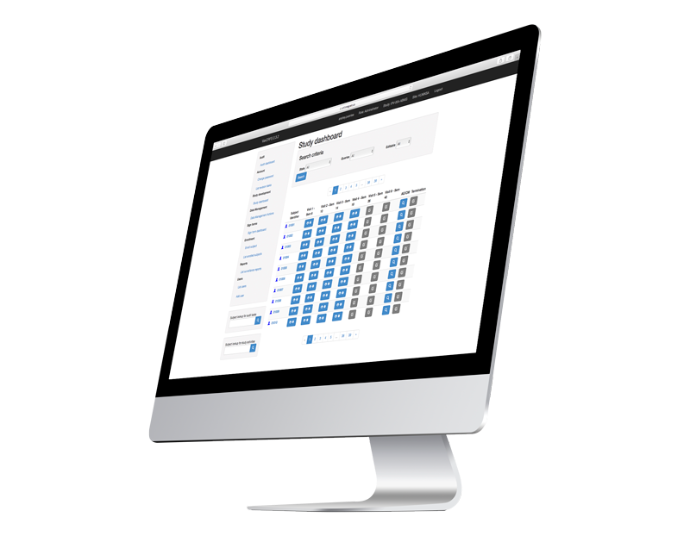

eCRF and Data management Experience
Using our eCRF and Services
:
-
Our eCRF uses CDASH standard and allows Data export in SAS format for Statistics Analysis. Also available in Excel and CSV.
-
Easy CDISC compliance due to CDASH and SDTM standard conversion according to study needs.
Completed:
-
Polio Phase 2 study - Colombia (Vax Trials - Bill & Melinda Gates Foundation) - 2014
-
Polio Phase 2 study - Panama, Dominican Republic (Vax Trials, FIDEC) - 2017
Ongoing:
-
Real World Evidence (RWE) birth control - Peru (JSS, Bayer) - 2020
-
Norovirus Observational study - Panama (Cevaxin, Takeda) - 2020
-
Breast Cancer (RWE) - Colombia (JSS, Pfizer) - 2020
-
Prostate Cancer (RWE) - Colombia (JSS, Bayer) - 2021
-
Sexual Arousal Disorder Phase 3 - Colombia (JSS, Litaphar) -2021

We develop solutions as a service, take a look at how they work.
CONTACT USCase Report Form System
CRF & Data Management Services
Our eCRF is a WEB EDC system developed in order to allow users to enter, verify, and sign clinical trials information, where the following functionalities are enabled.
Randomize Subjects
Enroll Subjects
Enter Data
Verify Information
Manage Queries
Add Electronic Signature (Pls)
Download Reports
The mentioned functionalities can be performed by the following roles, depending on the access specified for each one:
Data Entry
CRA
Private Investigator
Our eCRF is already integrated and synchronized with our PFS (Patient Follow Up System), used by sites to manage a clinical trial, thereby reducing errors and costs in data transfer or digitalization.
Role Dashboards
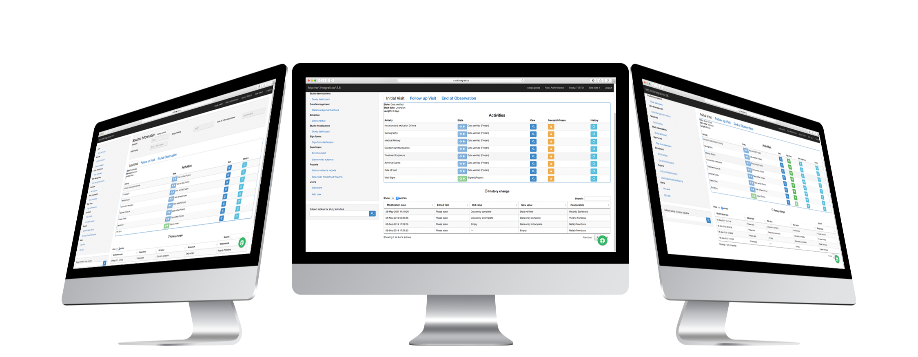
Data Entry Role
The person in charge of entering the information will have the Data Entry role. This role can:
-
Randomize Subjects
-
Enroll Subjects
-
Enter Data
CRA Role
The CRA is the person in charge of verifying and managing queries. This role can:
-
Verify Information
-
Manage Queries
-
Download reports
Principal Investigator
The principal investigator verifies the information and signs it for approval. This role can:
-
Add Electronic Signature (Pls)
-
Download reports
A User-Friendly Solution
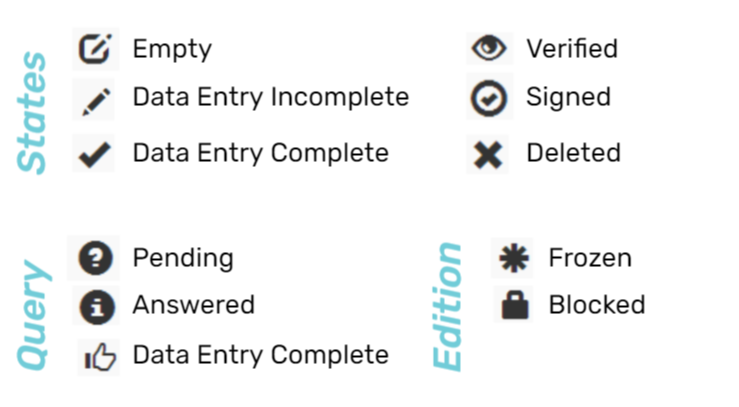
Colors and Icons
The status of the visits and observations are labeled with colors and icons according to the stage of each process and what is happening to it. The colors and icons are used as follows:
Empty page / No info
Information not
verified by CRA
Pending queries
Verified by the CRA
Signed by PI
If you want to know more about how our systems are validated and comply with 21 CFR Part 11
Reports
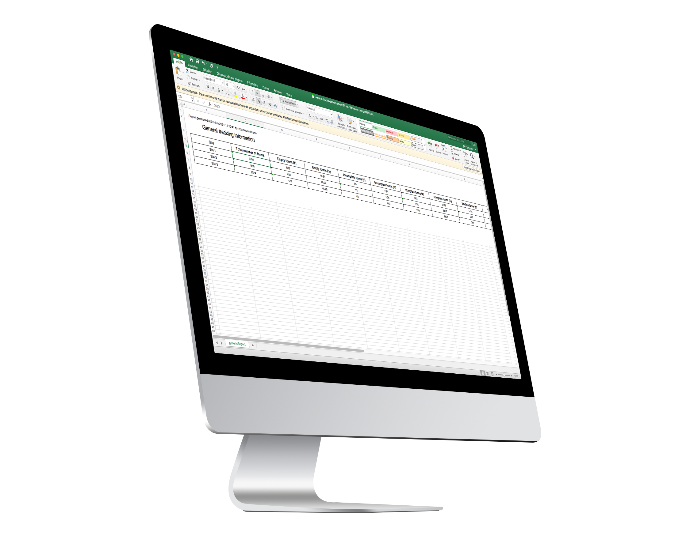
Surveillance Reports
Investigators and CRAs can generate the following surveillance reports to see how the Trial is progressing:
-
General Tracking Report
-
Query Tracking Report
-
Visit Report
-
Monitoring Visit Report
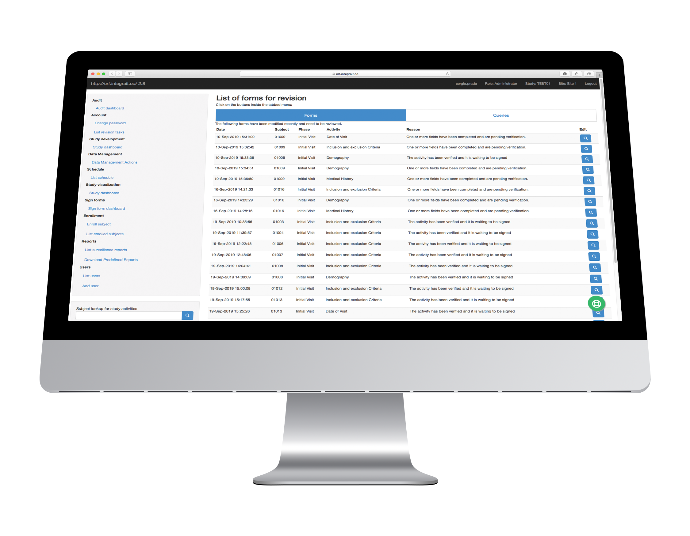
Predefined Reports
Administrators can also create reports with personalized information obtained from the databases for particular queries. These reports can be set up at the beginning or during the Trial on an on-demand basis, and at no additional cost.






