Multiple Solutions for your sponsors
We have a set of solutions that will allow the sponsors to have global control and the most relevant data of the studies they are sponsoring, all this with the necessary quality standards.
- TrialPal ePRO + Site CTMS
- EPI
- CRF (Case Report Form System & Data Management Services)
- Integration (eDC, eCOA, ePRO)
Want to know how to get our solutions?
Schedule a meeting with us!
Join our Partnership Program
With an initial study discount!
Join by clicking on the Apply Now button
Our Services For Sponsors
We have created a set of services and solutions to help the sponsor to follow-up all the studies, and also receive reports for the CROs to make real time decisions.
EPI
We develop solutions as a service, take a look at how they work.
CONTACT USTrialPal ePRO + PFS (Patient Follow Up System Site CTMS)
TrialPal + PFS II
An intuitive and easy-to-use tool that allows monitoring of study participants and the study in general, combined with an easy to use app created to keep track of your patients.

- We have a product that contains a differentiating factor that generates value, offering solutions for monitoring and defining processes. It informs you in the form of a dashboard, containing the information collected. Alerts and notifications are generated, and there is a projection that helps decision taking.
- PFS, allows the collection of all the data necessary for decision making, and the visualization of the traceability in changes, guaranteeing transparency of the information provided.
-
EPI
Covid 19 symptom tracking
It is a simple tool that allows you to comply with the biosafety protocols required by each country.
This allows you to manage the return to the new normal of your company employees through temperature recording surveys, among others.
With Epi, you can keep a record of all your collaborators, as well as those suspected and / or confirmed with COVID-19.
You can also geographically locate the report to know where the collaborator is at the time of recording and the risk level of the sector.
Additionally, thanks to the informative Dashboard, you will be able to know things such as the number of reports received and the symptoms reported by your collaborators.
If you want to know more about how our systems are validated and comply with 21 CFR Part 11
Do you want to talk and know more about any solution?
schedule a meetingCase Report
Form System
CRF & Data Management Services
Our WEB Information System (Case Report Form System) is used to enter and monitor the information of a clinical study efficiently, quickly and safely. It can be configured according to study and protocol requirement.
The data we store is protected so that only authorized persons can access it. Additionally, our data is backed up by backup copies that are saved every 6 hours.
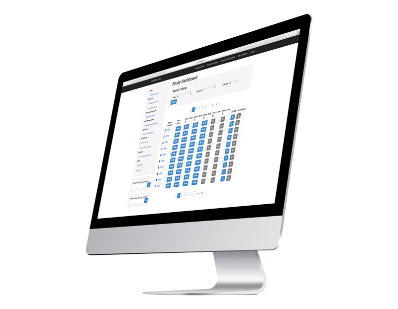

When working with our CRF, you will have total confidence in where your data is and who accesses it, since we work with processes with high quality standards and security. We have a team that will monitor your requirements and provide solutions to them.
You will have access to documents such as manuals, configuration of forms and validation of data. You can contact us to show you a DEMO and test the tool.

Integration between EDC, eCOA and Site CTMS
Our CRF, TrialPal & PFS or Trial 360 combined
The combination of three of our best solutions resulted in an easy to use platform for all the needs of your site.
Monitoring of study participants and the study in general, minimizing processes, reducing costs, keep a tracking on eDiaries and registers, further to monitoring the information in a clinical study; all in one integration.
Integration Model
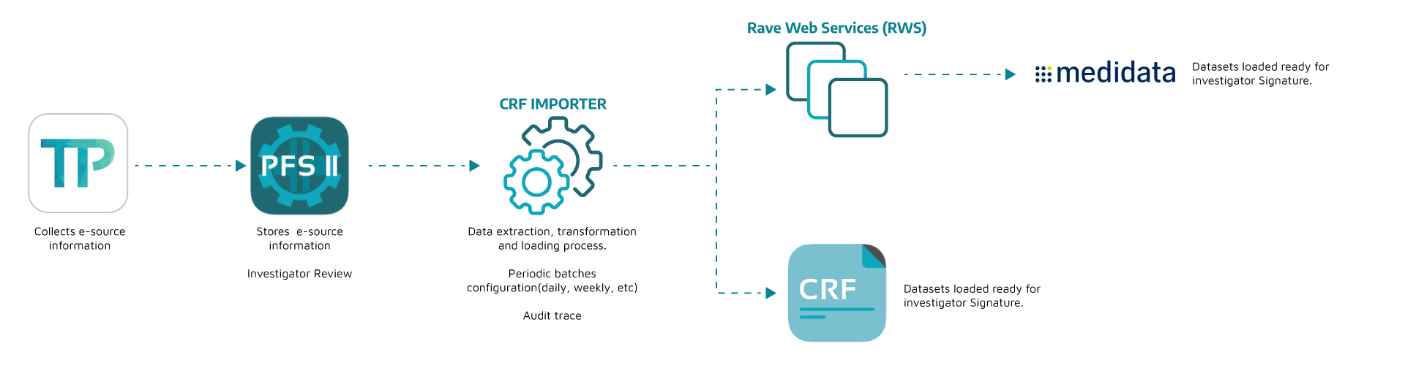
Notes:
-
Integrations take 2-3 weeks depending (approx) on study forms (# variables, visits, e-dairy days).
-
Testing and validation takes 1 week (approx), to make sure integration works flawlessly.
-
Rave dedicated user needed to authenticate transactions via RWS.
All digital, less time, save $
No more loss of information, no more hiring of digitization services, and no more human errors. Get savings. Allow remote access to CRAs. Be efficient
Customized stats
Real-Time with export to excel documents with customized stats and data
Get close to your patient or subject
Receive immediate notifications of any patient report, act quickly, gather insight and respond to study objectives. Use the end-user preferred communication method such as secure chat, schedule visits, and easily set up a new study within a couple of hours according to study protocol.
Security
All your data completely safe and systems 21 CRF Part 11 Compliant.
Click on each solution
If you want to know more about it

