
Multiple Solutions for your site
From a complete vertical Life Science ERP to a simple-to-use scheduling system and clinical trial management system, including an Electronic Health Record design for clinical trials, that can also be integrated with the most popular eCRF or EDC such as RAVE from Medidata.
- Trial 360 (a modular and complete Site CTMS and ERP)
- PFS (Patient Follow up System)
- eCRF (Case Report Form System & Data Management Services)
- Integration Services and API (Between EDC, eCOA & Site CTMS)
Want to know how to get our solutions?
Schedule a meeting with us!
Join our Partnership Program
With an initial study discount!
Join by clicking on the Apply Now button
Our Services For Sites
We have created a set of services and solutions to help Sites adapt to a digital world and transform with the accompaniment needed to change and gain insight and control to comply with each clinical trial objective. We have learned that a happy Site is the basis of an outstanding and efficient study.
We develop software as a service, take a look at how they work.
CONTACT USTrial 360
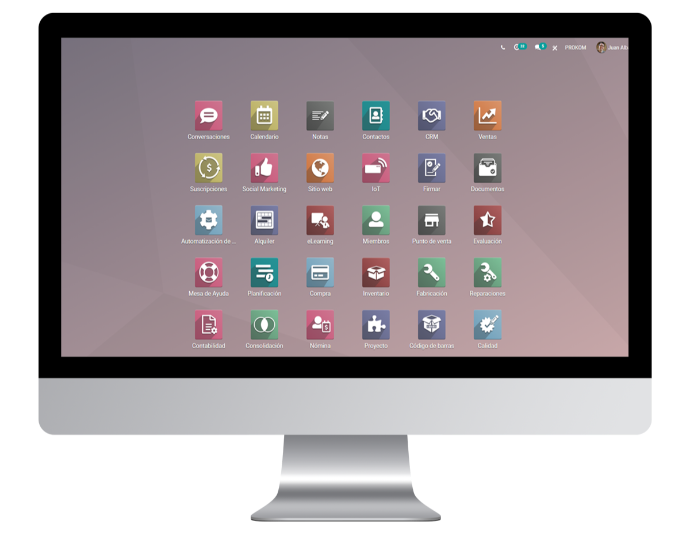
An All-in-one Ecosystem
Trial360 is a web based 21 CFR Part 11 compliant, easy to use platform, a vertical Life Science ERP and Site CTMS that speeds up processes and improves quality across the clinical study.
Patient Follow
up System
PFS
It is an intuitive and easy-to-use tool that allows monitoring of study participants and the study in general.
It is a configurable platform, in order to satisfy the needs of the project to the maximum, allowing the activation of the different modules and functions according to the requirements.
Follow-up can also be registered by the same participant through the TrialPal mobile application, where forms and surveys can be configured so that the user reports important information for the study; information that can be viewed from the PFS by users of the sites, either in a general or detailed manner.
PFS, allows the collection of all the data necessary for decision making, allowing the visualizationg of the traceability in changes, guaranteeing the transparency of the information provided.

Its main
characteristics are:
-
Configurable tool
-
Dynamism for the management of visits and follow-up to patients
-
Record of all activities and events of a subject
-
Traceability and auditing of all actions carried out on the platform
-
Use of Trial Pal mobile application to collect data in the first person
-
Download reports and information directly from the platform
If you want to know more about how our systems are validated and comply with 21 CFR Part 11
Do you want to chat and know more about any solution?
schedule a meeting
Integration between EDC, eCOA and Site CTMS
Our eCRF, TrialPal & PFS or Trial 360 combined
The combination of three of our best solutions resulted in an easy to use platform for all the needs of your site.
Monitoring of study participants and the study in general, minimizing processes, reducing costs, keep a tracking on eDiaries and registers, further to monitoring the information in a clinical study; all in one integration.
Integration Model
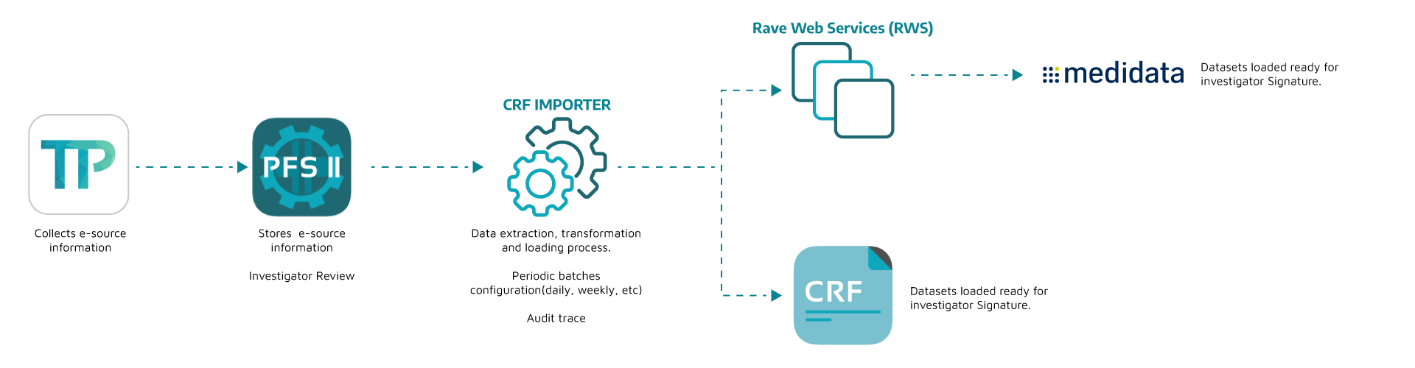
Notes:
-
Integrations take 2-3 weeks (approx) depending on study forms (# variables, visits, e-dairy days).
-
Testing and validation takes 1 week (approx), to ensure integration works flawlessly.
-
Rave dedicated user needed to authenticate transactions via RWS.
All digital, less time, save $
No more loss of information, no more hiring of digitization services, and no more human errors. Get savings. Allow remote access to CRAs. Be efficient
Customized stats
Real-Time with ablility to export to excel, containing customized stats and data
Get close to your patient or subject
Receive immediate notifications of any patient report, act quickly, gather insights and respond to study objectives. Use the end-user preferred communication method such as secure chat, schedule visits, and easily set up a new study within a couple of hours according to study protocol.
Security
All your data completely safe and systems 21 CRF Part 11 Compliant
Click on each solution
If you want to know more about it
If you want to know more about how our systems are validated and comply with 21 CRF Part 11
Study Cases
Grateful customers
At Integra IT we care about the satisfaction of our clients and the good performance of each of our solutions, always providing clients with the best support and listening to each request they may have.
Here are some of our study cases:


Cevaxin
Our solutions helped CEVAXIN to improve on its efficiency and management of data in every one of its clinical studies, helping it to make less mistakes and gather all the information digitally and securely.
University of Mainz
The university was in charge of one of the studies for Covid 19. Using our Winning Formula (PFS II and TrialPal), it was able to organize everything regarding the studies during pandemic times and to manage all the study results and data.
"The TrialPal Mobile App made it possible for us to continuously collect a large number of data sets from our study participants at a high frequency. Thanks to the Integra IT team, which reacted quickly and flexibly in a pandemic situation and created interfaces to our study systems in order to optimally integrate the app into our processes. Together, good progress has been made in vaccine development."



