Integration EDC, eCOA and Site CTMS
Our CRF, TrialPal & PFS or Trial 360 combined

Join our Partnership Program
With an initial study discount!
Join by clicking on the Apply Now button
Integration Model
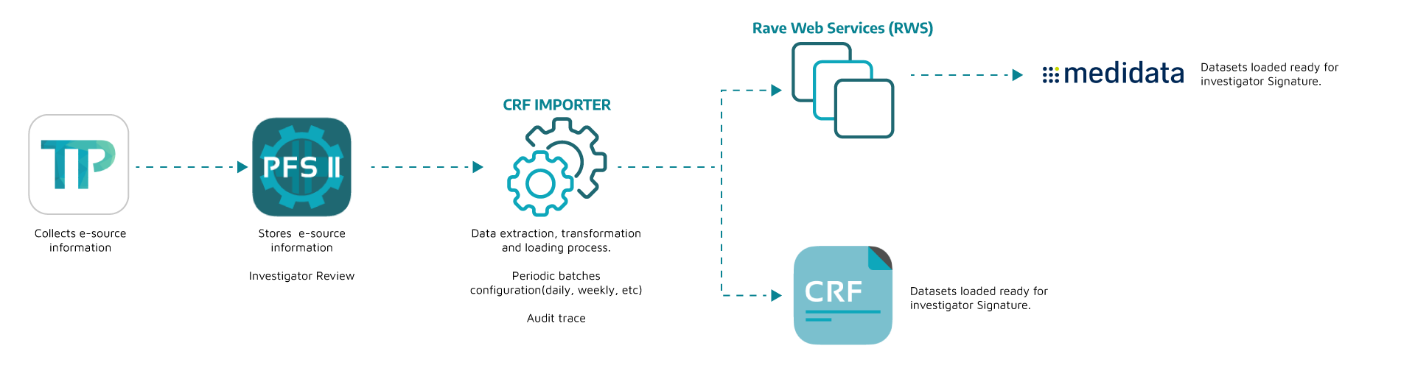
Notes:
-
Integrations take 2-3 weeks depending (approx) on study forms (# variables, visits, e-dairy days).
-
Testing and validation takes 1 week (approx), to make sure integration works flawless.
-
Rave dedicated user needed to authenticate transactions via RWS.
All digital, less time, save $
No more loss of information, no more hiring of digitization services, and no more human errors. Get savings. Allow remote access to CRAs. Be efficient
Customized stats
Real-Time with export to excel documents with customized stats and data
Get close to your patient or subject
Receive immediate notifications of any patient report, act quickly, gather insight and respond to study objectives. Use the end-user preferred communication method such as secure chat, schedule visits, and easily set up a new study within a couple of hours according to study protocol.
Security
All your data completely secured and systems 21 CFR Part 11 Compliant
Click on each solution
If you want to know more about it
If you want to know more about how our systems are validated and comply with 21 CFR Part 11

